WeLCOME
LIDEA
REGISTRY
Join our Endometriosis and Adenomyosis Registry: participate towards improved understanding, health, and building a stronger community.
WHAT IS the study about?
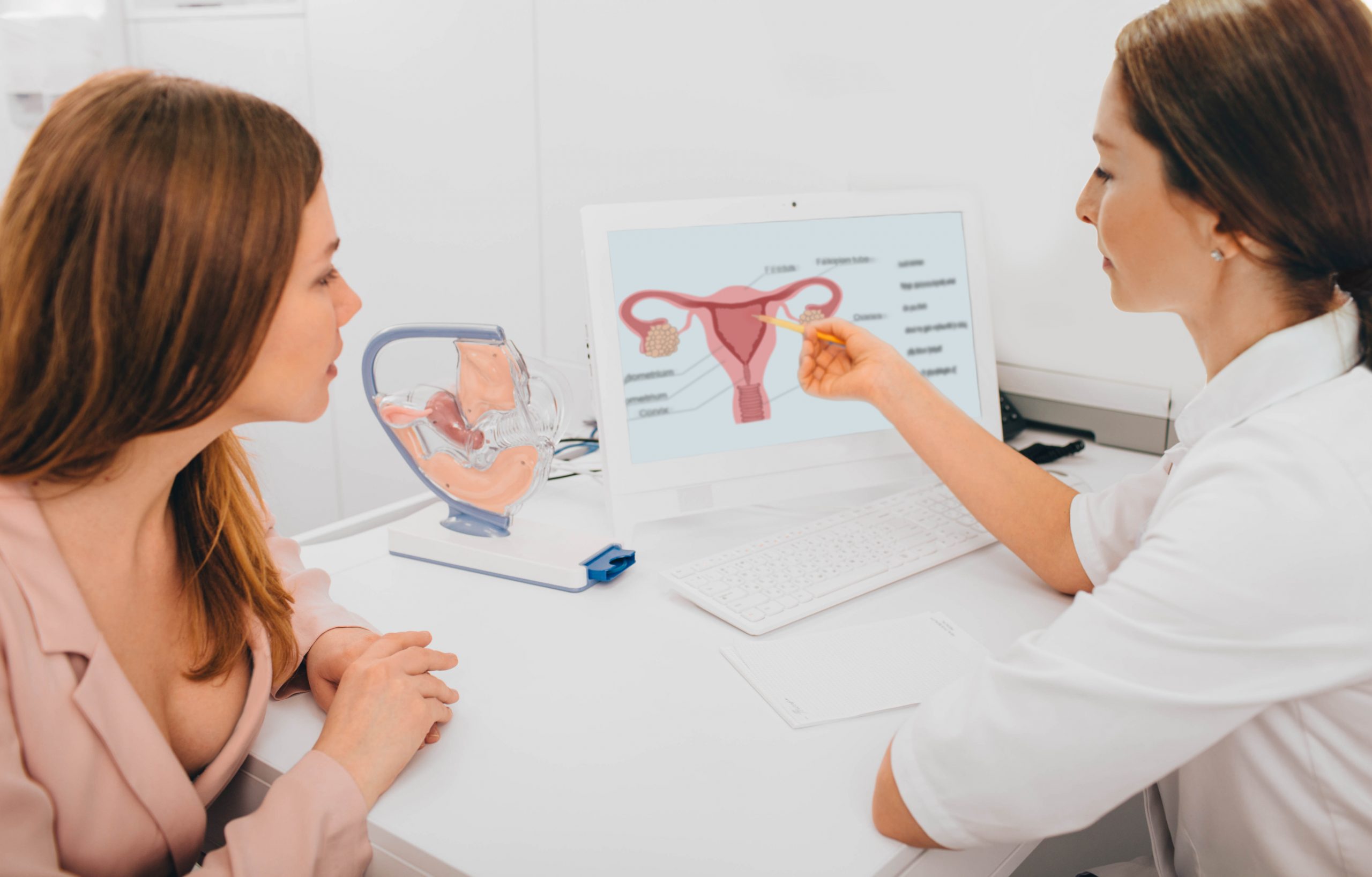
This study, the Long-Term International Dual Source Endometriosis and Adenomyosis Registry (LIDEA Registry), is about better understanding two medical conditions, endometriosis and adenomyosis and focuses on four key research themes:
- Pain and bleeding,
- Disease burden,
- Diagnostic pathways and
- Treatment effectiveness.
Overall, our goal is to improve the understanding and quality of life for individuals living with these conditions.
KEY GOALS for 2029
countries
participants
physicians
years

Why can i participate?
Your physician has identified you as someone who may be eligible for study participation. This study is open to individuals aged 14 years to 50 who have a confirmed or suspected diagnosis of endometriosis and/or adenomyosis.
Countries of recruitment include: Germany, France, Italy, Spain, United Kingdom and the United States.
What is expected when I decide to participate?
Step 1: Provide Consent
Provide your consent online or on paper as instructed by your physician.
Step 2: Sign-up in Greenlight Guru Clinical
Greenlight Guru Clinical is a web-based platform which will be used to complete the study questionnaires directly on your mobile phone, laptop or computer.
Step 3: Study Enrollment!
You are now enrolled in the study and you will receive the study questionnaires via email and SMS as shown in the Questionnaire Schedule below.
Study Enrollment
Your personal information including name, mobile phone number and e-mail address will need to be entered in Greenlight Guru Clinical. After signing up, you will see your first questionnaire, including questions about you (age, weight, medical history), your endometriosis and/or adenomyosis, quality-of-life, and experience of pain. This questionnaire will take you approximately 30-40 minutes to answer.
... 2 weeks later
Two weeks after study entry, you will be invited to start answering a daily pain diary. The dairy needs to be completed every day for 30 consecutive days. The daily questionnaire will take between 2-5 minutes to answer each day.
...6 Months later
You will receive a first follow-up questionnaire at approximately 6 months after study entry.
The follow-up questionnaire will include specific questions related to your symptoms, treatment, and medication history as well as quality of life, and pain. This questionnaire will require approximately 30-40 minutes to answer.
A second daily pain dairy will be activated and needs to be completed for 30 consecutive days. The daily questionnaire will take you 2-5 minutes to answer each day.
...12 Months later
You will receive a second follow-up questionnaire at approximately 12 months after study entry.
The follow-up questionnaire will include specific questions related to your symptoms, treatment, and medication history as well as quality of life, and pain. This questionnaire will require approximately 30-40 minutes to answer.
A final daily pain dairy will be activated and needs to be completed for 30 consecutive days. The daily questionnaire will take you 2-5 minutes to answer each day.
...24 Months later
You will receive a third follow-up questionnaire at approximately 24 months after study entry.
The follow-up questionnaire will include specific questions related to your symptoms, treatment, and medication history as well as quality of life, and pain. This questionnaire will require approximately 30-40 minutes to answer.
...36 Months later
You will receive a final follow-up questionnaire at approximately 36 months after study entry.
The follow-up questionnaire will include specific questions related to your symptoms, treatment, and medication history as well as quality of life, and pain. This questionnaire will require approximately 30-40 minutes to answer.
faqs

Why do you need my contact data?
Contact details you provide us will only be used to reach you in connection with this study.
Questionnaires and remuneration for your time will be sent directly to you via email and/or text message.
If a response to a questionnaire remains missing or is unclear, you will receive an email and text message and might be contacted via phone by the local study team.
If your contact details change during the study, reach out to our study team.
Do I receive a study treatment?
No.
No medications are provided to you by the study sponsor or study team. You will continue to receive the treatment your physician deems appropriate.
Are there any risks when I participate?
No.
Because this study involves completing web-based questionnaires, it is highly unlikely that you would be injured by participating. However, if you become ill or physically injured during this study, please contact your physician immediately. Since you are not taking a study drug, the sponsor makes no commitment to provide compensation in the event of injury due to your taking part in this study.
When does my study participation end?
Your study participation ends when you completed all study questionnaires.
Your participation in the study is voluntary. If you no longer wish to participate in the study, you can withdraw your consent at any time without giving reasons. In this case your name and contact data will be deleted. We only use the data provided to us up to the point of withdrawal of consent. In case of study withdrawal, we will not contact you further. Your participation to this study will end when you have completed all study questionnaires and if all missing information has been collected. Your consent from the study can be withdrawn by contacting the project team. The study team or the sponsor can also stop this study (or your participation in this study) at any time.
Do I get a remuneration for participating?
As a thank you for your participation, you will receive a Tremendous voucher of $20 for completion of the baseline and follow-up questionnaires (6, 12, 24, and 36 months).
For the daily pain diary, you will receive a $40 Tremendous voucher if you completed a minimum of 21 days out of 30 days (70% of data provided) at baseline, 6 months, and 12 months.
Vouchers will be send directly via email upcon completion of the questionnaire or 30-day diary.
Yes, i would like to participate!
Sign-up in the LIDEA Registry study via the QR code provided by your physician.
We are excited to have you as a member in our study!